How Do You Get Endometriosis Part 1: The Cell
Endometriosis takes many steps to form into the fully established disease we know. No, you didn’t just “catch endo” like the flu, even if it felt like symptoms came on fast and hard. There are so many steps in developing endo that it’s much more like a story unfolding, and a BIG story at that. Still, this story has a clear beginning: The creation of an endometriosis-like (endo-like) cell. These cells are similar to the cells of the endometrial lining, but distinctly different… and not in a good way.
Endo-like cells are more like jacked Navy SEALs than cushions of fertility. They are often highly sensitive to estrogen and very resistant to progesterone, meaning there’s lots of growth without much cooling. They can avoid normal cellular death and immune cleanup processes, and become pain factories. These cells are great at migrating, self-healing, and invading into the smallest nooks and crannies (which may be associated with the start of adenomyosis in the uterine wall)(1). Where did this thing come from? Turns out, research has ‘a great many ideas.
Meet Your Endometrial Cells
Having endo-like cells within the uterus helps explain why the normally placed endometrium lining may not be so normal to begin with in someone with endo (17-19) Having them placed outside the uterus helps explain pain with first menses, certain types of adenomyosis, and even in rare instances in men!
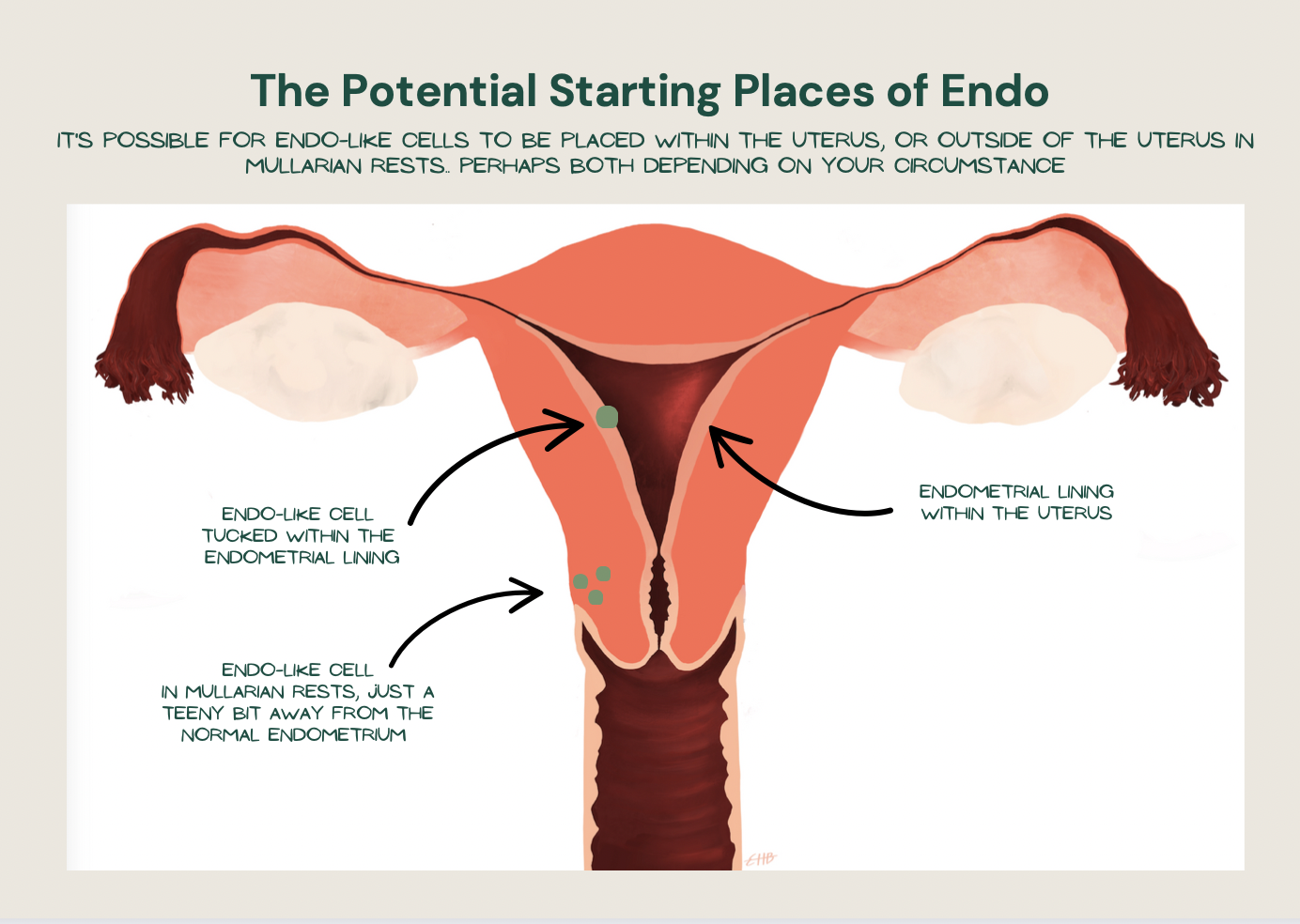
Endometrial cells line the inside of the uterus, making up your endometrial lining, or endometrium. This lining grows each month in preparation for a baby to be implanted, and, if no baby comes, shed with menses.
After shedding, this tissue regrows! We don’t give it enough credit for doing this. Like, wouldn’t it be great if fingers re-grew if they were cut off? They don’t, but your endometrial cells do. This is thanks to the stem cells within that allow your normal endometrial cells to have this superpower. In fact, for the sake of this endo story, we could think of these cells as superheroes.
Normally, these cells should exist solely within the uterus. However, it’s totally possible for endometrial cells to be born a hop, skip, and a jump outside of the uterus, in something called Müllerian rests. Not a problem in healthy individuals…. although obviously a problem if those cells start behaving like endo.
Training Endometrial Cells To Become Endometriosis Supervillains
In the case of endo, it appears some of these endometrial cells are behaving quite differently. How?
Genetically, you inherit a mixed bag of cellular behaviors, some of which will be stuck with you for life. But, here’s a pop quiz: If corn has 32,000 genes and a tree has 45,000, how many genes do humans have? Only about 25,000! Fewer than corn.
It’s possible for humans have less genes than corn (or trees, for that matter) thanks to epigenetics. Epigenetics is how genes can change their behavior depending on what “information” they pick up. In fact, gene behaviors can turn “on” or “off” based on any number of lifestyle experiences you have throughout your entire life, including stress levels, diet, sun exposure, community level, toxins, and more! This is how a measly 25,000 genes can change their behavior so profoundly from person to person, allowing us to have fewer genes yet be so complex. These epigenetic changes can also be beneficial, or not… mostly the latter when it comes to endometriosis.
Research has pinpointed numerous genetic and epigenetic alterations that create a supervillain out of your superhero endometrial cell, becoming the highly feared endo-like cell. Dun, dun, dunnnn. Here are examples of some contributors:
Genetics and endometriosis: Shake What Your Mama Gave You
To start, it’s estimated that a little over half of us (51% of us, to be exact) may be born with the genes that predispose us to develop endo. For example, a protein-coding gene called BCL6 may set our endometrial cells up for progesterone resistance. Another gene (NPSR1) may contribute to some of the pain and inflammation we face. Gene inheritance is how endo can run in families, as in, your more likely to develop endo if your mother had it.
But, even though you have have inherited “endo genes”, it doesn’t mean your normal endometrial cells will automatically become supervillains, just that you have a lot more likelihood of it happening. You also can develop endo even if it doesn’t run in your family. This is how genetics is one factor of many in “getting endo”, but it’s not a genetic disease. (2-4)
Endometriosis and Chemical Exposure
Dioxins are one of the most feared environmental contaminants worldwide. They are nasty byproducts of industrial and farming processes that linger in the environment for decades, known to be potent endocrine disruptors and linked to a variety of diseases, including endometriosis.
Endometrial cells exposed to dioxins can get their behavior scrambled, so-to-speak, in ways that prevent normal cell death—something you really want when it comes to endo. Dioxins can also contribute to progesterone resistance and create abnormalities with the Homeobox A10 gene, which effects fertility. And, because the epigenetic effects of dioxins are so potent, there is concern that these can be passed down inter-generationally (meaning you could have inherited these epigenetic changes from your mother or grandmother who was exposed, even if you yourself weren’t). (5-6)
Most ALL bath, both, laundry, household, and beauty care will have phthalates in it. If you’re not actively avoiding it, it will be in your products. Not fair, but you can make changes!
Phthalates are used to make plastics durable and fragrances last longer, they’re found in everything from synthetic floor tiles and carpets to lipstick, and your favorite smelling shampoo, body wash, and perfume. Yes, even if organic or “all natural” (because of a loophole, if you see fragrance/parfum as an ingredient it almost certainly has phthalates in it).
When normal endometrial cells are exposed to phthalates they not only show signs of inflammation and oxidative stress, but they also become more invasive and proliferative, much like the behavior of an endo-like cell.
Unfortunately, there are 13 different studies demonstrating those with endometriosis have higher levels of phthalates in their blood, urine, or peritoneal fluid (the slippery, immune rich liquid that lines your peritoneal cavity) than those without endo. We’re smothered, and our endometrial cells may be paying a high price. (7-8)
Bisphenol A, commonly called BPA, is used primarily to make plastic products, as well as acting as protective coating for things like food and drink cans, hot coffee cups, and thermal receipt paper. Itʻs basically everywhere. Exposing normal endometrial cells to BPA has been shown to significantly decrease progesterone receptors, creating an estrogen-dominant endometrial cell that is apt to grow, grow, grow, without any cooling. (9-11)
Endometriosis and Chronic Inflammation
In one study, nearly half of women with endometriosis were found to have a chronic infection of the uterine lining, perhaps contributing to the creation of an endo-like cell. (20)
When chronic inflammation ensues, it damages our cellular ability to get oxygen. To survive this suffocation, our normal endometrial cells have a last-ditch epigenetic survival tactic: turning into a mesenchymal cell, a type of stem cell that has even more superpowers than usual. Unfortunately, with power comes supervillain attitude, and these new endometrial mesenchymal cells may start to adopt even more endo-like behaviors: increased invasiveness into small nooks and crannies, an enhanced ability to migrate around the body, and even more resistance to cellular death.
This is why chronic inflammation may be another prerequisite for the early development of an aggressive endo-like cell. (12-13)
Why would your endometrial cells be suffocating? There are numerous facets linked to endo I’ll discuss in following blogs, including bacterial overgrowths in the reproductive tract or endo belly dysbiosis (both biggies for creating chronic inflammation with endo!), chemical exposure, excess blood in the peritoneal cavity (from too heavy menses combined with retrograde flow, or established endo lesions once they take hold), lack of antioxidants, not enough physical activity, and more. Once endo is established, it will also contribute to the equation.
Creating Different Types of Endometriosis
The unique combination of
the genes we were born with, plus
the epigenetic behaviors they accrue over time
are the main reason why we develop an endo-like supervillain in the first place. These very different combinations also explain how there can be so many different types of endo—perhaps up to 65 different types! Really? Yes. These different types have different behaviors, for example:
While most types of endometriosis regress during pregnancy, other can progress.
Some types of endometriosis grow slowly and may barely spread, while others grow and spread at a rapid rate.
Certain types of deep endometriosis lesions are associated with cancer, while others are not.
There are a wide variety of inflammation levels around what appear to be similar types of lesions, meaning that the immune reaction is widely diverse among types.
Progesterone resistance in endo types vary from non-existent to very pronounced.
While most endo requires estrogens to grow, some can develop without much estrogen, as seen in men or women more than 10 years after menopause (who aren’t taking hormones).
The wide variety of differing behaviors also helps us understand how endo effects our bodies differently! For example, why there’s such a wide array of symptoms experienced by endo sufferers, why different treatment options work well for some people and not at all for others, and why we benefit from unique treatment plans rather than the same blanket treatment strategy for all types. (14-15)
Retraining Endometriosis: A Treatment Strategy to Add in
What if we removed some of these factors? Worth a shot!
Genetics and epigenetics allow us to understand many things about endo, perhaps most importantly reminding us that endometriosis is no one’s fault. Genetics and epigenetics remind us that a myriad of factors contribute to each case of endo, many factors that are out of our control. Indeed you may have been born with endo genes, in addition to inheriting epigenetic alterations from your grandma who was exposed to dioxins, in addition to being exposed to copious amounts of phthalates and BPA throughout your childhood. Officially: out of our control.
However, some if this information is within our control as of now, and this is where hope shines through. Know that epigenetic expression is not written in stone. Just as negative inputs can train supervillain cellular behavior, so too can positive inputs flip them back toward superhero.
While that’s not to say we simply flip off the epigenetic “switch” for endo (akin to having a cure)—at least not yet—studying epigenetics is enormously encouraging since it suggests we may be able to change the way our endo behaves. By removing triggers known to increase the likelihood of endo behavior (chronic inflammation of the uterus or peritoneal cavity, BPA, pthalates, dioxins, and other endocrine disrupting chemicals), we may be able to give our body a better fighting chance to rid this invader.
This was demonstrated in a recent study on people living with an autoimmune disease called ulcerative colitis (an inflammatory bowel disease). When the participants applied a diet called the Paleo Autoimmune Protocol, which focuses on both nutrient infusion and inflammatory food trigger removal, they found that after 6 weeks a total of 324 “bad” genes associated with the disease had epigenetically changed for the better (!!!) while the inflammatory responses had decreased significantly. One patient went into full clinical remission.(21)
So, what if we could change the epigenetic alterations in our endo that to allow for normal cellular death (supporting our body in killing this abnormal cell)? What if we could swap out estrogen sensitivity or progesterone resistance to foster normal hormonal receptivity, stopping the rapid growth of lesions without any cooling? By changing endo behavior, we may stand a fighting chance.
Science is right there, with brand new endo research recommending that clinicians focus on the “prevention of additional genetic or epigenetic incidents, by reducing environmental pollution and by reducing the [inflammation]” to better manage endo. (16)
Stay tuned for next week, where we dive into HOW just HOW to start avoiding these epigenetic “bad guys”!
1 Guo S. W. (2020). The Pathogenesis of Adenomyosis vis-à-vis Endometriosis. Journal of clinical medicine, 9(2), 485. https://doi.org/10.3390/jcm9020485
2 Hansen, K. A., & Eyster, K. M. (2010). Genetics and genomics of endometriosis. Clinical obstetrics and gynecology, 53(2), 403–412. https://doi.org/10.1097/GRF.0b013e3181db7ca1
3 Yoo, J. Y., Kim, T. H., Fazleabas, A. T., Palomino, W. A., Ahn, S. H., Tayade, C., Schammel, D. P., Young, S. L., Jeong, J. W., & Lessey, B. A. (2017). KRAS Activation and over-expression of SIRT1/BCL6 Contributes to the Pathogenesis of Endometriosis and Progesterone Resistance. Scientific reports, 7(1), 6765. https://doi.org/10.1038/s41598-017-04577-w
4 Tapmeier, T. T., Rahmioglu, N., Lin, J., De Leo, B., Obendorf, M., Raveendran, M., Fischer, O. M., Bafligil, C., Guo, M., Harris, R. A., Hess-Stumpp, H., Laux-Biehlmann, A., Lowy, E., Lunter, G., Malzahn, J., Martin, N. G., Martinez, F. O., Manek, S., Mesch, S., Montgomery, G. W., … Zondervan, K. T. (2021). Neuropeptide S receptor 1 is a nonhormonal treatment target in endometriosis. Science translational medicine, 13(608), eabd6469. https://doi.org/10.1126/scitranslmed.abd6469
5 Shmarakov I. O. (2015). Retinoid-xenobiotic interactions: the Ying and the Yang. Hepatobiliary surgery and nutrition, 4(4), 243–267. https://doi.org/10.3978/j.issn.2304-3881.2015.05.05
6 Bruner-Tran, K. L., Ding, T., & Osteen, K. G. (2010). Dioxin and endometrial progesterone resistance. Seminars in reproductive medicine, 28(1), 59–68. https://doi.org/10.1055/s-0029-1242995; Koukoura, O., Sifakis, S., & Spandidos, D. A. (2016). DNA methylation in endometriosis. Molecular medicine reports, 13(4), 2939–2948. https://doi.org/10.3892/mmr.2016.4925
7 Chou, Y. C., & Tzeng, C. R. (2021). The impact of phthalate on reproductive function in women with endometriosis. Reproductive medicine and biology, 20(2), 159–168. https://doi.org/10.1002/rmb2.12364
8 Chou, Y. C., & Tzeng, C. R. (2021). The impact of phthalate on reproductive function in women with endometriosis. Reproductive medicine and biology, 20(2), 159–168. https://doi.org/10.1002/rmb2.12364
9 Aldad, T. S., Rahmani, N., Leranth, C., & Taylor, H. S. (2011). Bisphenol-A exposure alters endometrial progesterone receptor expression in the nonhuman primate. Fertility and sterility, 96(1), 175–179. https://doi.org/10.1016/j.fertnstert.2011.04.010
10 Signorile PG, Spugnini EP, Mita L, Mellone P, D’Avino A, Bianco M, Diano N, Caputo L, Rea F, Viceconte R, Portaccio M, Viggiano E, Citro G, Pierantoni R, Sica V, Vincenzi B, Mita DG, Baldi F, Baldi A. (2010) Pre-natal exposure of mice to bisphenol A elicits an endometriosis-like phenotype in female offspring. Gen Comp Endocrinol. 168(3), 318-25. https://www. doi.org/10.1016/j.ygcen.2010.03.030
11 Hiroi, H., Tsutsumi, O., Takeuchi, T., Momoeda, M., Ikezuki, Y., Okamura, A., Yokota, H., & Taketani, Y. (2004). Differences in serum bisphenol a concentrations in premenopausal normal women and women with endometrial hyperplasia. Endocrine journal, 51(6), 595–600. https://doi.org/10.1507/endocrj.51.595
12 Wu, M. H., Hsiao, K. Y., & Tsai, S. J. (2019). Hypoxia: The force of endometriosis. The journal of obstetrics and gynaecology research, 45(3), 532–541. https://doi.org/10.1111/jog.13900
13 Kalluri, R., & Weinberg, R. A. (2009). The basics of epithelial-mesenchymal transition. The Journal of clinical investigation, 119(6), 1420–1428. https://doi.org/10.1172/JCI39104
14 Martin DC. Endometriosis Concepts and Theories. Resurge Press, Richmond, Virginia, revised August 6, 2021. https://www.danmartinmd.com/endoconcepts.html. Accessed 2/1/21
15 Koninckx, P. R., Ussia, A., Adamyan, L., Wattiez, A., Gomel, V., & Martin, D. C. (2020). Correction: Heterogeneity of endometriosis lesions requires individualisation of diagnosis and treatment and a different approach to research and evidence based medicine. Facts, views & vision in ObGyn, 11(3), 263.
16 Koninckx, P. R., Ussia, A., Adamyan, L., Wattiez, A., Gomel, V., & Martin, D. C. (2020). Correction: Heterogeneity of endometriosis lesions requires individualisation of diagnosis and treatment and a different approach to research and evidence based medicine. Facts, views & vision in ObGyn, 11(3), 263
17 Nayyar, A., Saleem, M. I., Yilmaz, M., DeFranco, M., Klein, G., Elmaliki, K. M., Kowalsky, E., Chatterjee, P. K., Xue, X., Viswanathan, R., Shih, A. J., Gregersen, P. K., & Metz, C. N. (2020). Menstrual effluent provides a novel diagnostic window on the pathogenesis of Endometriosis. Frontiers in Reproductive Health, 2. https://doi.org/10.3389/frph.2020.00003;
18 Noble, L. S., Takayama, K., Zeitoun, K. M., Putman, J. M., Johns, D. A., Hinshelwood, M. M., Agarwal, V. R., Zhao, Y., Carr, B. R., & Bulun, S. E. (1997). Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. The Journal of clinical endocrinology and metabolism, 82(2), 600–606. https://doi.org/10.1210/jcem.82.2.3783;
19 Liu, H., & Lang, J. H. (2011). Is abnormal eutopic endometrium the cause of endometriosis? The role of eutopic endometrium in pathogenesis of endometriosis. Medical science monitor : international medical journal of experimental and clinical research, 17(4), RA92–RA99. https://doi.org/10.12659/msm.881707
20 Cicinelli, E., Trojano, G., Mastromauro, M., Vimercati, A., Marinaccio, M., Mitola, P. C., Resta, L., & de Ziegler, D. (2017). Higher prevalence of chronic endometritis in women with endometriosis: a possible etiopathogenetic link. Fertility and sterility, 108(2), 289–295.e1. https://doi.org/10.1016/j.fertnstert.2017.05.016
21 Chandrasekaran, A., Molparia, B., Akhtar, E., Wang, X., Lewis, J. D., Chang, J. T., Oliveira, G., Torkamani, A., & Konijeti, G. G. (2019). The Autoimmune Protocol Diet Modifies Intestinal RNA Expression in Inflammatory Bowel Disease. Crohn's & colitis 360, 1(3), otz016. https://doi.org/10.1093/crocol/otz016